Page 130 - Instant notes
P. 130
Physical chemistry 116
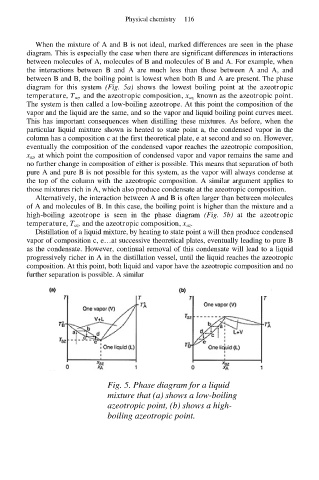
When the mixture of A and B is not ideal, marked differences are seen in the phase
diagram. This is especially the case when there are significant differences in interactions
between molecules of A, molecules of B and molecules of B and A. For example, when
the interactions between B and A are much less than those between A and A, and
between B and B, the boiling point is lowest when both B and A are present. The phase
diagram for this system (Fig. 5a) shows the lowest boiling point at the azeotropic
temperature, T az, and the azeotropic composition, x az, known as the azeotropic point.
The system is then called a low-boiling azeotrope. At this point the composition of the
vapor and the liquid are the same, and so the vapor and liquid boiling point curves meet.
This has important consequences when distilling these mixtures. As before, when the
particular liquid mixture shown is heated to state point a, the condensed vapor in the
column has a composition c at the first theoretical plate, e at second and so on. However,
eventually the composition of the condensed vapor reaches the azeotropic composition,
x az, at which point the composition of condensed vapor and vapor remains the same and
no further change in composition of either is possible. This means that separation of both
pure A and pure B is not possible for this system, as the vapor will always condense at
the top of the column with the azeotropic composition. A similar argument applies to
those mixtures rich in A, which also produce condensate at the azeotropic composition.
Alternatively, the interaction between A and B is often larger than between molecules
of A and molecules of B. In this case, the boiling point is higher than the mixture and a
high-boiling azeotrope is seen in the phase diagram (Fig. 5b) at the azeotropic
temperature, T az, and the azeotropic composition, x az.
Distillation of a liquid mixture, by heating to state point a will then produce condensed
vapor of composition c, e…at successive theoretical plates, eventually leading to pure B
as the condensate. However, continual removal of this condensate will lead to a liquid
progressively richer in A in the distillation vessel, until the liquid reaches the azeotropic
composition. At this point, both liquid and vapor have the azeotropic composition and no
further separation is possible. A similar
Fig. 5. Phase diagram for a liquid
mixture that (a) shows a low-boiling
azeotropic point, (b) shows a high-
boiling azeotropic point.