Page 129 - Instant notes
P. 129
Phase diagrams of mixtures 115
. It should be noted that metal alloys are often completely miscible
ideal solid solutions of two metals, and so their solid-liquid phase diagrams are often
similar in form to this diagram. In this case it is a liquid mixture rather than a vapor
mixture which is the phase seen at high temperature in the phase diagram (Fig. 3), and a
solid mixture rather than a liquid mixture which is the phase at low temperature. A
mixture with a state point such as c is then in the two-phase (solid and liquid) region, and
d and e give the composition of the liquid and solid respectively.
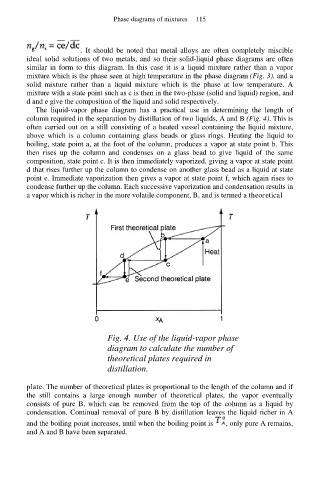
The liquid-vapor phase diagram has a practical use in determining the length of
column required in the separation by distillation of two liquids, A and B (Fig. 4). This is
often carried out on a still consisting of a heated vessel containing the liquid mixture,
above which is a column containing glass beads or glass rings. Heating the liquid to
boiling, state point a, at the foot of the column, produces a vapor at state point b. This
then rises up the column and condenses on a glass bead to give liquid of the same
composition, state point c. It is then immediately vaporized, giving a vapor at state point
d that rises further up the column to condense on another glass bead as a liquid at state
point e. Immediate vaporization then gives a vapor at state point f, which again rises to
condense further up the column. Each successive vaporization and condensation results in
a vapor which is richer in the more volatile component, B, and is termed a theoretical
Fig. 4. Use of the liquid-vapor phase
diagram to calculate the number of
theoretical plates required in
distillation.
plate. The number of theoretical plates is proportional to the length of the column and if
the still contains a large enough number of theoretical plates, the vapor eventually
consists of pure B, which can be removed from the top of the column as a liquid by
condensation. Continual removal of pure B by distillation leaves the liquid richer in A
and the boiling point increases, until when the boiling point is , only pure A remains,
and A and B have been separated.