Page 135 - Instant notes
P. 135
Ions in aqueous solution 121
Thermodynamic properties
Salts dissolve producing both cations and anions, which means that it is impossible to
measure thermodynamic data for individual ion types. The solvation of ions from the gas
phase (the enthalpy of solvation, see Topic B2) is a thermodynamically favorable
exothermic process, which increases the stability of the ions in solution and gives out
energy. However, in order for the enthalpy of solution (∆H sol, see Topic B2) to be
exothermic, this enthalpy of solution must be greater than the lattice enthalpy; otherwise
the overall reaction is endothermic.
The entropy change due to breaking up the salt into its constituent gaseous ions is
also positive, but in addition there is an entropy term due to ion solvation. Once the ion is
solvated, the water molecules in the solvation shell are arranged around each ion with a
center of symmetry in the system (Fig. 1). They are relatively tightly packed with respect
to liquid water and hence have lower entropy and occupy a lower volume. In contrast, in
the bulk solution, the water molecules are extensively hydrogen bonded (see Topic H6) in
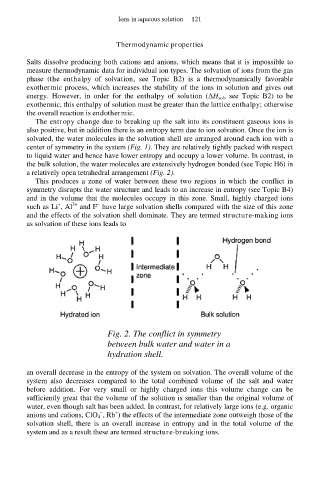
a relatively open tetrahedral arrangement (Fig. 2).
This produces a zone of water between these two regions in which the conflict in
symmetry disrupts the water structure and leads to an increase in entropy (see Topic B4)
and in the volume that the molecules occupy in this zone. Small, highly charged ions
+
−
3+
such as Li , Al and F have large solvation shells compared with the size of this zone
and the effects of the solvation shell dominate. They are termed structure-making ions
as solvation of these ions leads to
Fig. 2. The conflict in symmetry
between bulk water and water in a
hydration shell.
an overall decrease in the entropy of the system on solvation. The overall volume of the
system also decreases compared to the total combined volume of the salt and water
before addition. For very small or highly charged ions this volume change can be
sufficiently great that the volume of the solution is smaller than the original volume of
water, even though salt has been added. In contrast, for relatively large ions (e.g. organic
−
+
anions and cations, ClO 4 , Rb ) the effects of the intermediate zone outweigh those of the
solvation shell, there is an overall increase in entropy and in the total volume of the
system and as a result these are termed structure-breaking ions.