Page 157 - Instant notes
P. 157
Macroscopic aspects of ionic motion 143
Ions in aqueous solution (E2)
(E1)
Conductance and conductivity
When an electric field is put across an aqueous ionic solution by applying a voltage
between two parallel plate electrodes, the cations are attracted towards the negative plate
(cathode) and the anions towards the positive plate (anode).
This movement of ions in a field is called migration and the movement of charge
results in a current in the electric circuit connecting the electrodes. The field between the
plates is initially linear and produces a constant ion velocity at all points. However, if
there is no redox reaction, cations and anions will separate and collect at the cathode and
anode respectively with time, balancing the electrode charge and there will be fewer ions
in the bulk of solution. This is a process known as concentration polarization, which
also leads to modification of the original linear potential profile between the two
electrodes and a variation of ion velocity between the plates. The observed current will
also fall with time (as at infinite time, the ion flow will have stopped as the ions will have
reached the plates and the current will have decreased to zero). Concentration
polarization is therefore useful when separating ions is desirable, such as in
electrophoresis and electro-osmosis, but this is to be avoided when measuring
fundamental ion motion.
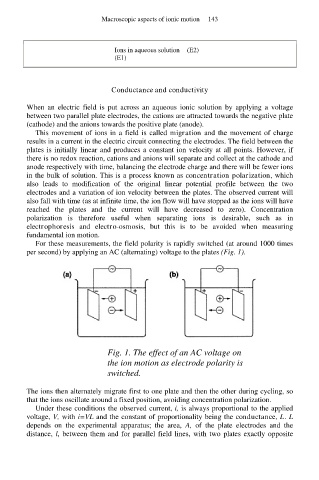
For these measurements, the field polarity is rapidly switched (at around 1000 times
per second) by applying an AC (alternating) voltage to the plates (Fig. 1).
Fig. 1. The effect of an AC voltage on
the ion motion as electrode polarity is
switched.
The ions then alternately migrate first to one plate and then the other during cycling, so
that the ions oscillate around a fixed position, avoiding concentration polarization.
Under these conditions the observed current, i, is always proportional to the applied
voltage, V, with i=VL and the constant of proportionality being the conductance, L. L
depends on the experimental apparatus; the area, A, of the plate electrodes and the
distance, l, between them and for parallel field lines, with two plates exactly opposite