Page 154 - Instant notes
P. 154
Physical chemistry 140
(see Topic C2). The cell Nernst equation can then be used to calculate the difference
between the cell potential in the standard state, when pH=0, and the biological standard
−
state, when pH=7 (see Topic B2). The RH electrode has a constant concentration of Cl
and is a reference electrode (see Topic E4); the first two terms on the right-hand side of
the equation are therefore constant and the cell potential could be used to measure
changes in the pH of the solution in the LH half-cell. In fact, using the hydrogen gas
electrode to measure pH is impractical (for the same reasons as the standard hydrogen
electrode (SHE) is impractical, see Topic E4). Instead, solution pH measurements are
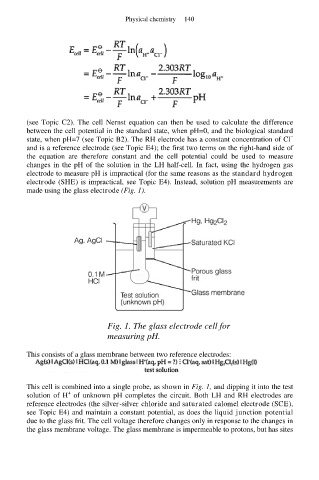
made using the glass electrode (Fig. 1).
Fig. 1. The glass electrode cell for
measuring pH.
This consists of a glass membrane between two reference electrodes:
This cell is combined into a single probe, as shown in Fig. 1, and dipping it into the test
+
solution of H of unknown pH completes the circuit. Both LH and RH electrodes are
reference electrodes (the silver-silver chloride and saturated calomel electrode (SCE),
see Topic E4) and maintain a constant potential, as does the liquid junction potential
due to the glass frit. The cell voltage therefore changes only in response to the changes in
the glass membrane voltage. The glass membrane is impermeable to protons, but has sites