Page 159 - Instant notes
P. 159
Macroscopic aspects of ionic motion 145
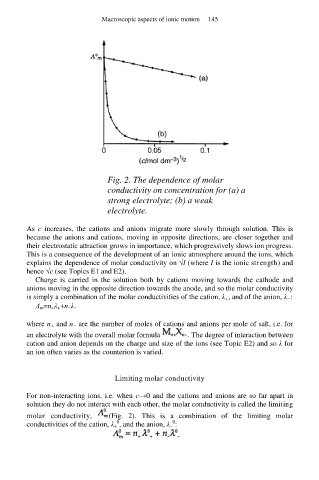
Fig. 2. The dependence of molar
conductivity on concentration for (a) a
strong electrolyte; (b) a weak
electrolyte.
As c increases, the cations and anions migrate more slowly through solution. This is
because the anions and cations, moving in opposite directions, are closer together and
their electrostatic attraction grows in importance, which progressively slows ion progress.
This is a consequence of the development of an ionic atmosphere around the ions, which
explains the dependence of molar conductivity on √I (where I is the ionic strength) and
hence √c (see Topics E1 and E2).
Charge is carried in the solution both by cations moving towards the cathode and
anions moving in the opposite direction towards the anode, and so the molar conductivity
is simply a combination of the molar conductivities of the cation, λ +, and of the anion, λ −:
Λ m=n +λ ++n −λ −
where n + and n − are the number of moles of cations and anions per mole of salt, i.e. for
an electrolyte with the overall molar formula . The degree of interaction between
cation and anion depends on the charge and size of the ions (see Topic E2) and so λ for
an ion often varies as the counterion is varied.
Limiting molar conductivity
For non-interacting ions, i.e. when c→0 and the cations and anions are so far apart in
solution they do not interact with each other, the molar conductivity is called the limiting
molar conductivity, (Fig. 2). This is a combination of the limiting molar
0
0
conductivities of the cation, λ + , and the anion, λ − :