Page 211 - Instant notes
P. 211
The kinetics of real systems 197
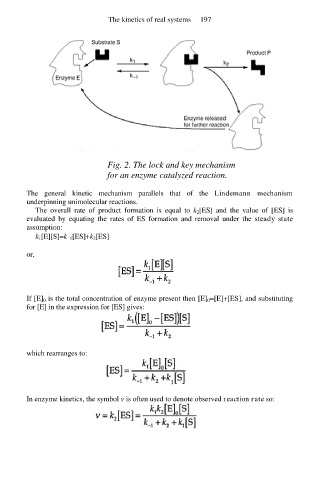
Fig. 2. The lock and key mechanism
for an enzyme catalyzed reaction.
The general kinetic mechanism parallels that of the Lindemann mechanism
underpinning unimolecular reactions.
The overall rate of product formation is equal to k 2[ES] and the value of [ES] is
evaluated by equating the rates of ES formation and removal under the steady state
assumption:
k 1[E][S]=k −1[ES]+k 2[ES]
or,
If [E] 0 is the total concentration of enzyme present then [E] 0=[E]+[ES], and substituting
for [E] in the expression for [ES] gives:
which rearranges to:
In enzyme kinetics, the symbol v is often used to denote observed reaction rate so: