Page 262 - Instant notes
P. 262
Physical chemistry 248
rather than continuing with the 3d elements, because of the effects of shielding and
penetration (Topic G6).
The analogous electron configurations confer the periodicity on the physical and
chemical properties of the elements on which the structure of the periodic table was
originally based.
Atomic radii
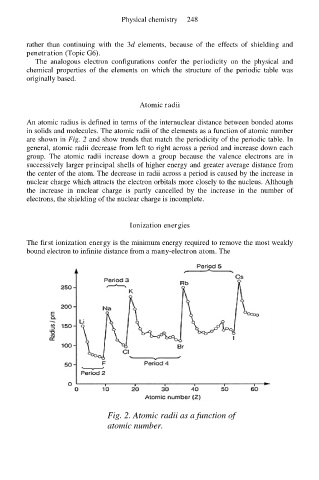
An atomic radius is defined in terms of the internuclear distance between bonded atoms
in solids and molecules. The atomic radii of the elements as a function of atomic number
are shown in Fig. 2 and show trends that match the periodicity of the periodic table. In
general, atomic radii decrease from left to right across a period and increase down each
group. The atomic radii increase down a group because the valence electrons are in
successively larger principal shells of higher energy and greater average distance from
the center of the atom. The decrease in radii across a period is caused by the increase in
nuclear charge which attracts the electron orbitals more closely to the nucleus. Although
the increase in nuclear charge is partly cancelled by the increase in the number of
electrons, the shielding of the nuclear charge is incomplete.
Ionization energies
The first ionization energy is the minimum energy required to remove the most weakly
bound electron to infinite distance from a many-electron atom. The
Fig. 2. Atomic radii as a function of
atomic number.