Page 263 - Instant notes
P. 263
Chemical and structural effects of quantization 249
second ionization energy is the minimum energy required to remove the next electron
from the singly charged cation, and so on.
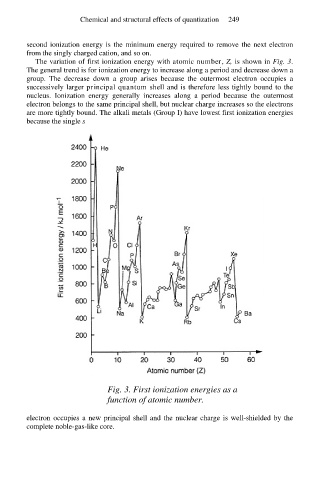
The variation of first ionization energy with atomic number, Z, is shown in Fig. 3.
The general trend is for ionization energy to increase along a period and decrease down a
group. The decrease down a group arises because the outermost electron occupies a
successively larger principal quantum shell and is therefore less tightly bound to the
nucleus. Ionization energy generally increases along a period because the outermost
electron belongs to the same principal shell, but nuclear charge increases so the electrons
are more tightly bound. The alkali metals (Group I) have lowest first ionization energies
because the single s
Fig. 3. First ionization energies as a
function of atomic number.
electron occupies a new principal shell and the nuclear charge is well-shielded by the
complete noble-gas-like core.