Page 283 - Instant notes
P. 283
Valence bond theory 269
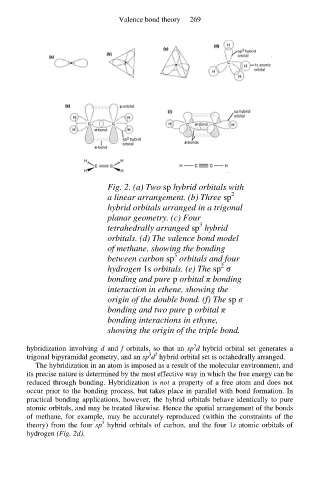
Fig. 2. (a) Two sp hybrid orbitals with
2
a linear arrangement. (b) Three sp
hybrid orbitals arranged in a trigonal
planar geometry. (c) Four
3
tetrahedrally arranged sp hybrid
orbitals. (d) The valence bond model
of methane, showing the bonding
3
between carbon sp orbitals and four
2
hydrogen 1s orbitals. (e) The sp σ
bonding and pure p orbital π bonding
interaction in ethene, showing the
origin of the double bond. (f) The sp σ
bonding and two pure p orbital π
bonding interactions in ethyne,
showing the origin of the triple bond.
3
hybridization involving d and f orbitals, so that an sp d hybrid orbital set generates a
3 2
trigonal bipyramidal geometry, and an sp d hybrid orbital set is octahedrally arranged.
The hybridization in an atom is imposed as a result of the molecular environment, and
its precise nature is determined by the most effective way in which the free energy can be
reduced through bonding. Hybridization is not a property of a free atom and does not
occur prior to the bonding process, but takes place in parallel with bond formation. In
practical bonding applications, however, the hybrid orbitals behave identically to pure
atomic orbitals, and may be treated likewise. Hence the spatial arrangement of the bonds
of methane, for example, may be accurately reproduced (within the constraints of the
3
theory) from the four sp hybrid orbitals of carbon, and the four 1s atomic orbitals of
hydrogen (Fig. 2d).