Page 278 - Instant notes
P. 278
Physical chemistry 264
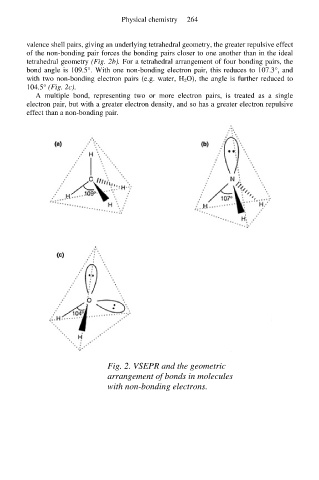
valence shell pairs, giving an underlying tetrahedral geometry, the greater repulsive effect
of the non-bonding pair forces the bonding pairs closer to one another than in the ideal
tetrahedral geometry (Fig. 2b). For a tetrahedral arrangement of four bonding pairs, the
bond angle is 109.5°. With one non-bonding electron pair, this reduces to 107.3°, and
with two non-bonding electron pairs (e.g. water, H 2O), the angle is further reduced to
104.5° (Fig. 2c).
A multiple bond, representing two or more electron pairs, is treated as a single
electron pair, but with a greater electron density, and so has a greater electron repulsive
effect than a non-bonding pair.
Fig. 2. VSEPR and the geometric
arrangement of bonds in molecules
with non-bonding electrons.