Page 277 - Instant notes
P. 277
Elementary valence theory 263
bonding, will adopt a geometry which will minimize repulsive forces by maximizing the
distances between pairs.
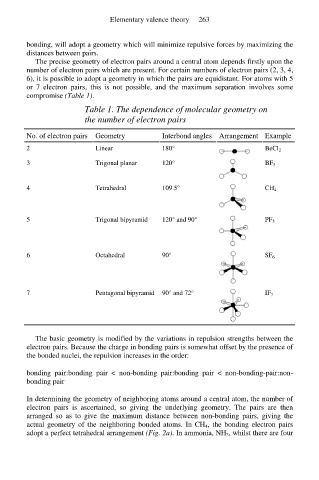
The precise geometry of electron pairs around a central atom depends firstly upon the
number of electron pairs which are present. For certain numbers of electron pairs (2, 3, 4,
6), it is possible to adopt a geometry in which the pairs are equidistant. For atoms with 5
or 7 electron pairs, this is not possible, and the maximum separation involves some
compromise (Table 1).
Table 1. The dependence of molecular geometry on
the number of electron pairs
No. of electron pairs Geometry Interbond angles Arrangement Example
2 Linear 180° BeCl 2
3 Trigonal planar 120° BF 3
4 Tetrahedral 109.5° CH 4
5 Trigonal bipyramid 120° and 90° PF 5
6 Octahedral 90° SF 6
7 Pentagonal bipyramid 90° and 72° IF 7
The basic geometry is modified by the variations in repulsion strengths between the
electron pairs. Because the charge in bonding pairs is somewhat offset by the presence of
the bonded nuclei, the repulsion increases in the order:
bonding pair:bonding pair < non-bonding pair:bonding pair < non-bonding-pair:non-
bonding pair
In determining the geometry of neighboring atoms around a central atom, the number of
electron pairs is ascertained, so giving the underlying geometry. The pairs are then
arranged so as to give the maximum distance between non-bonding pairs, giving the
actual geometry of the neighboring bonded atoms. In CH 4, the bonding electron pairs
adopt a perfect tetrahedral arrangement (Fig. 2a). In ammonia, NH 3, whilst there are four