Page 276 - Instant notes
P. 276
Physical chemistry 262
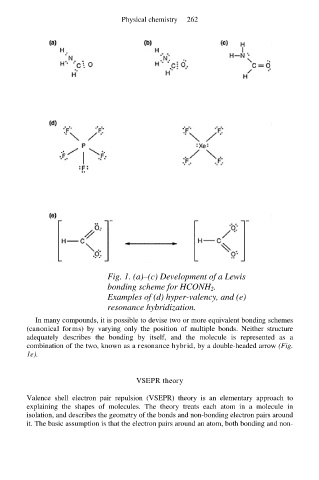
Fig. 1. (a)–(c) Development of a Lewis
bonding scheme for HCONH 2.
Examples of (d) hyper-valency, and (e)
resonance hybridization.
In many compounds, it is possible to devise two or more equivalent bonding schemes
(canonical forms) by varying only the position of multiple bonds. Neither structure
adequately describes the bonding by itself, and the molecule is represented as a
combination of the two, known as a resonance hybrid, by a double-headed arrow (Fig.
1e).
VSEPR theory
Valence shell electron pair repulsion (VSEPR) theory is an elementary approach to
explaining the shapes of molecules. The theory treats each atom in a molecule in
isolation, and describes the geometry of the bonds and non-bonding electron pairs around
it. The basic assumption is that the electron pairs around an atom, both bonding and non-