Page 281 - Instant notes
P. 281
Valence bond theory 267
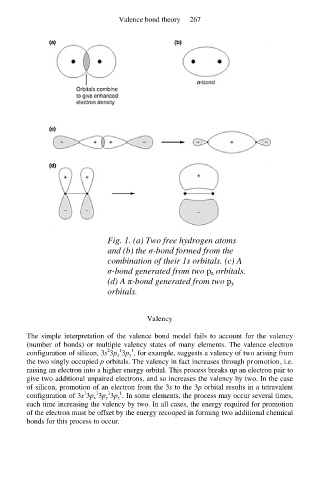
Fig. 1. (a) Two free hydrogen atoms
and (b) the σ-bond formed from the
combination of their 1s orbitals. (c) A
σ-bond generated from two p z orbitals.
(d) A π-bond generated from two p y
orbitals.
Valency
The simple interpretation of the valence bond model fails to account for the valency
(number of bonds) or multiple valency states of many elements. The valence electron
1
1
2
configuration of silicon, 3s 3p x 3p y , for example, suggests a valency of two arising from
the two singly occupied p orbitals. The valency in fact increases through promotion, i.e.
raising an electron into a higher energy orbital. This process breaks up an electron pair to
give two additional unpaired electrons, and so increases the valency by two. In the case
of silicon, promotion of an electron from the 3s to the 3p orbital results in a tetravalent
1
1
1
1
configuration of 3s 3p x 3p y 3p z . In some elements, the process may occur several times,
each time increasing the valency by two. In all cases, the energy required for promotion
of the electron must be offset by the energy recouped in forming two additional chemical
bonds for this process to occur.