Page 272 - Instant notes
P. 272
Physical chemistry 258
rotational degrees of freedom (from rotation about each of two axes perpendicular to the
main axis of the molecule and to one another). Any vibration contributes R to the
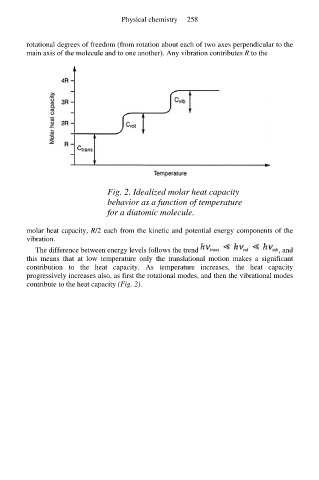
Fig. 2. Idealized molar heat capacity
behavior as a function of temperature
for a diatomic molecule.
molar heat capacity, R/2 each from the kinetic and potential energy components of the
vibration.
The difference between energy levels follows the trend , and
this means that at low temperature only the translational motion makes a significant
contribution to the heat capacity. As temperature increases, the heat capacity
progressively increases also, as first the rotational modes, and then the vibrational modes
contribute to the heat capacity (Fig. 2).