Page 367 - Instant notes
P. 367
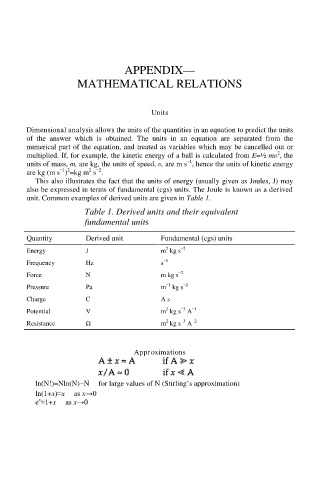
APPENDIX—
MATHEMATICAL RELATIONS
Units
Dimensional analysis allows the units of the quantities in an equation to predict the units
of the answer which is obtained. The units in an equation are separated from the
numerical part of the equation, and treated as variables which may be cancelled out or
2
multiplied. If, for example, the kinetic energy of a ball is calculated from E=½ mυ , the
−1
units of mass, m, are kg, the units of speed, υ, are m s , hence the units of kinetic energy
2 −2
−1 2
are kg (m s ) =kg m s .
This also illustrates the fact that the units of energy (usually given as Joules, J) may
also be expressed in terms of fundamental (cgs) units. The Joule is known as a derived
unit. Common examples of derived units are given in Table 1.
Table 1. Derived units and their equivalent
fundamental units
Quantity Derived unit Fundamental (cgs) units
2
−2
Energy J m kg s
−1
Frequency Hz s
−2
Force N m kg s
−2
−1
Pressure Pa m kg s
Charge C A s
−3
−1
2
Potential V m kg s A
−3
−2
2
Resistance Ω m kg s A
Approximations
ln(N!)=Nln(N)−N for large values of N (Stirling’s approximation)
ln(1+x)≈x as x→0
x
e ≈1+x as x→0