Page 364 - Instant notes
P. 364
Physical Chemistry 350
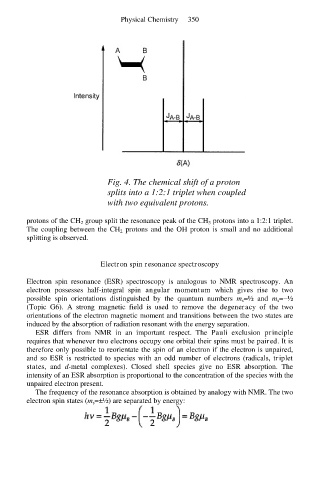
Fig. 4. The chemical shift of a proton
splits into a 1:2:1 triplet when coupled
with two equivalent protons.
protons of the CH 2 group split the resonance peak of the CH 3 protons into a 1:2:1 triplet.
The coupling between the CH 2 protons and the OH proton is small and no additional
splitting is observed.
Electron spin resonance spectroscopy
Electron spin resonance (ESR) spectroscopy is analogous to NMR spectroscopy. An
electron possesses half-integral spin angular momentum which gives rise to two
possible spin orientations distinguished by the quantum numbers m s=½ and m s=−½
(Topic G6). A strong magnetic field is used to remove the degeneracy of the two
orientations of the electron magnetic moment and transitions between the two states are
induced by the absorption of radiation resonant with the energy separation.
ESR differs from NMR in an important respect. The Pauli exclusion principle
requires that whenever two electrons occupy one orbital their spins must be paired. It is
therefore only possible to reorientate the spin of an electron if the electron is unpaired,
and so ESR is restricted to species with an odd number of electrons (radicals, triplet
states, and d-metal complexes). Closed shell species give no ESR absorption. The
intensity of an ESR absorption is proportional to the concentration of the species with the
unpaired electron present.
The frequency of the resonance absorption is obtained by analogy with NMR. The two
electron spin states (m s=±½) are separated by energy: