Page 360 - Instant notes
P. 360
Physical Chemistry 346
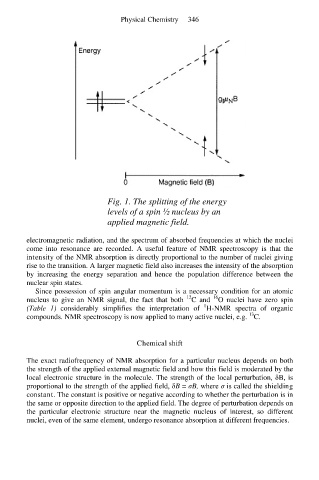
Fig. 1. The splitting of the energy
levels of a spin ½ nucleus by an
applied magnetic field.
electromagnetic radiation, and the spectrum of absorbed frequencies at which the nuclei
come into resonance are recorded. A useful feature of NMR spectroscopy is that the
intensity of the NMR absorption is directly proportional to the number of nuclei giving
rise to the transition. A larger magnetic field also increases the intensity of the absorption
by increasing the energy separation and hence the population difference between the
nuclear spin states.
Since possession of spin angular momentum is a necessary condition for an atomic
16
12
nucleus to give an NMR signal, the fact that both C and O nuclei have zero spin
1
(Table 1) considerably simplifies the interpretation of H-NMR spectra of organic
13
compounds. NMR spectroscopy is now applied to many active nuclei, e.g. C.
Chemical shift
The exact radiofrequency of NMR absorption for a particular nucleus depends on both
the strength of the applied external magnetic field and how this field is moderated by the
local electronic structure in the molecule. The strength of the local perturbation, δB, is
proportional to the strength of the applied field, δB = σB, where σ is called the shielding
constant. The constant is positive or negative according to whether the perturbation is in
the same or opposite direction to the applied field. The degree of perturbation depends on
the particular electronic structure near the magnetic nucleus of interest, so different
nuclei, even of the same element, undergo resonance absorption at different frequencies.