Page 363 - Instant notes
P. 363
Magnetic resonance spectroscopy 349
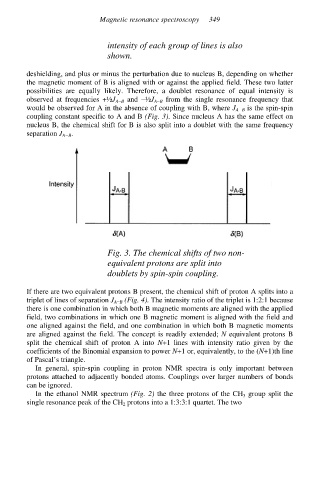
intensity of each group of lines is also
shown.
deshielding, and plus or minus the perturbation due to nucleus B, depending on whether
the magnetic moment of B is aligned with or against the applied field. These two latter
possibilities are equally likely. Therefore, a doublet resonance of equal intensity is
observed at frequencies +½J A−B and −½J A−B from the single resonance frequency that
would be observed for A in the absence of coupling with B, where J A−B is the spin-spin
coupling constant specific to A and B (Fig. 3). Since nucleus A has the same effect on
nucleus B, the chemical shift for B is also split into a doublet with the same frequency
separation J A−B.
Fig. 3. The chemical shifts of two non-
equivalent protons are split into
doublets by spin-spin coupling.
If there are two equivalent protons B present, the chemical shift of proton A splits into a
triplet of lines of separation J A−B (Fig. 4). The intensity ratio of the triplet is 1:2:1 because
there is one combination in which both B magnetic moments are aligned with the applied
field, two combinations in which one B magnetic moment is aligned with the field and
one aligned against the field, and one combination in which both B magnetic moments
are aligned against the field. The concept is readily extended; N equivalent protons B
split the chemical shift of proton A into N+1 lines with intensity ratio given by the
coefficients of the Binomial expansion to power N+1 or, equivalently, to the (N+1)th line
of Pascal’s triangle.
In general, spin-spin coupling in proton NMR spectra is only important between
protons attached to adjacently bonded atoms. Couplings over larger numbers of bonds
can be ignored.
In the ethanol NMR spectrum (Fig. 2) the three protons of the CH 3 group split the
single resonance peak of the CH 2 protons into a 1:3:3:1 quartet. The two