Page 355 - Instant notes
P. 355
Photochemistry in the real world 341
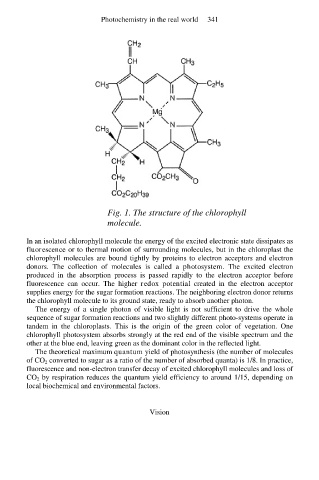
Fig. 1. The structure of the chlorophyll
molecule.
In an isolated chlorophyll molecule the energy of the excited electronic state dissipates as
fluorescence or to thermal motion of surrounding molecules, but in the chloroplast the
chlorophyll molecules are bound tightly by proteins to electron acceptors and electron
donors. The collection of molecules is called a photosystem. The excited electron
produced in the absorption process is passed rapidly to the electron acceptor before
fluorescence can occur. The higher redox potential created in the electron acceptor
supplies energy for the sugar formation reactions. The neighboring electron donor returns
the chlorophyll molecule to its ground state, ready to absorb another photon.
The energy of a single photon of visible light is not sufficient to drive the whole
sequence of sugar formation reactions and two slightly different photo-systems operate in
tandem in the chloroplasts. This is the origin of the green color of vegetation. One
chlorophyll photosystem absorbs strongly at the red end of the visible spectrum and the
other at the blue end, leaving green as the dominant color in the reflected light.
The theoretical maximum quantum yield of photosynthesis (the number of molecules
of CO 2 converted to sugar as a ratio of the number of absorbed quanta) is 1/8. In practice,
fluorescence and non-electron transfer decay of excited chlorophyll molecules and loss of
CO 2 by respiration reduces the quantum yield efficiency to around 1/15, depending on
local biochemical and environmental factors.
Vision