Page 351 - Instant notes
P. 351
Electronic spectroscopy 337
vibrations of the upper state (Fig. 2). The S nomenclature stands for singlet state and
refers to the fact that the ground states of most molecules contain paired electron spins
( ), which can adopt only one orientation with respect to an external magnetic field.
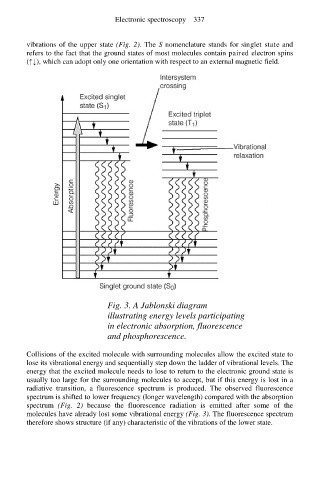
Fig. 3. A Jablonski diagram
illustrating energy levels participating
in electronic absorption, fluorescence
and phosphorescence.
Collisions of the excited molecule with surrounding molecules allow the excited state to
lose its vibrational energy and sequentially step down the ladder of vibrational levels. The
energy that the excited molecule needs to lose to return to the electronic ground state is
usually too large for the surrounding molecules to accept, but if this energy is lost in a
radiative transition, a fluorescence spectrum is produced. The observed fluorescence
spectrum is shifted to lower frequency (longer wavelength) compared with the absorption
spectrum (Fig. 2) because the fluorescence radiation is emitted after some of the
molecules have already lost some vibrational energy (Fig. 3). The fluorescence spectrum
therefore shows structure (if any) characteristic of the vibrations of the lower state.