Page 346 - Instant notes
P. 346
Physical Chemistry 332
If a molecule has a center of symmetry then no vibration can be both
Raman and infrared active. (A mode may be inactive in both.) If there is
no center of symmetry then some, but not necessarily all, vibrations may
be both Raman and infrared active.
The three vibrational modes of H 2O (no center of symmetry) are all both Raman and
infrared active.
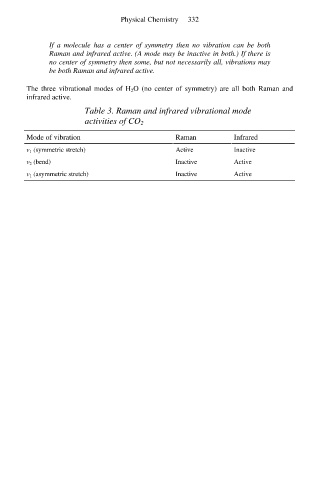
Table 3. Raman and infrared vibrational mode
activities of CO 2
Mode of vibration Raman Infrared
v 1 (symmetric stretch) Active Inactive
v 2 (bend) Inactive Active
v 1 (asymmetric stretch) Inactive Active