Page 344 - Instant notes
P. 344
Physical Chemistry 330
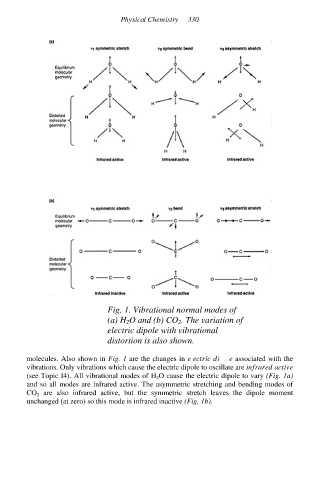
Fig. 1. Vibrational normal modes of
(a) H 2O and (b) CO 2. The variation of
electric dipole with vibrational
distortion is also shown.
molecules. Also shown in Fig. 1 are the changes in electric dipole associated with the
vibrations. Only vibrations which cause the electric dipole to oscillate are infrared active
(see Topic I4). All vibrational modes of H 2O cause the electric dipole to vary (Fig. 1a)
and so all modes are infrared active. The asymmetric stretching and bending modes of
CO 2 are also infrared active, but the symmetric stretch leaves the dipole moment
unchanged (at zero) so this mode is infrared inactive (Fig. 1b).