Page 339 - Instant notes
P. 339
Vibrational spectroscopy 325
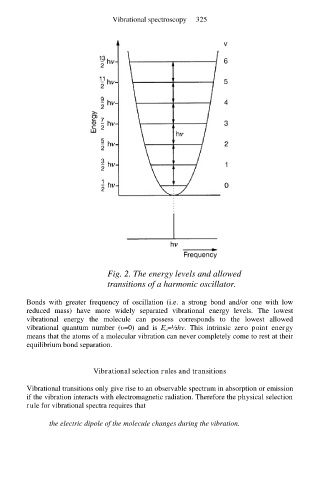
Fig. 2. The energy levels and allowed
transitions of a harmonic oscillator.
Bonds with greater frequency of oscillation (i.e. a strong bond and/or one with low
reduced mass) have more widely separated vibrational energy levels. The lowest
vibrational energy the molecule can possess corresponds to the lowest allowed
vibrational quantum number (υ=0) and is E υ=½hν. This intrinsic zero point energy
means that the atoms of a molecular vibration can never completely come to rest at their
equilibrium bond separation.
Vibrational selection rules and transitions
Vibrational transitions only give rise to an observable spectrum in absorption or emission
if the vibration interacts with electromagnetic radiation. Therefore the physical selection
rule for vibrational spectra requires that
the electric dipole of the molecule changes during the vibration.