Page 337 - Instant notes
P. 337
Vibrational spectroscopy 323
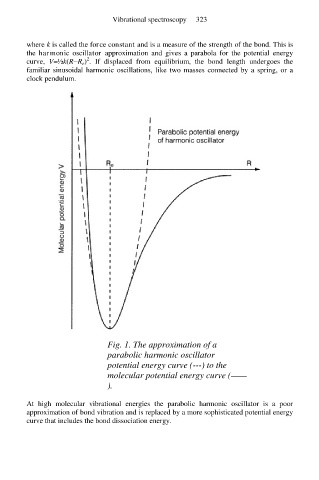
where k is called the force constant and is a measure of the strength of the bond. This is
the harmonic oscillator approximation and gives a parabola for the potential energy
2
curve, V=½k(R−R e) . If displaced from equilibrium, the bond length undergoes the
familiar sinusoidal harmonic oscillations, like two masses connected by a spring, or a
clock pendulum.
Fig. 1. The approximation of a
parabolic harmonic oscillator
potential energy curve (---) to the
molecular potential energy curve (——
).
At high molecular vibrational energies the parabolic harmonic oscillator is a poor
approximation of bond vibration and is replaced by a more sophisticated potential energy
curve that includes the bond dissociation energy.