Page 334 - Instant notes
P. 334
Physical Chemistry 320
The polarizability of a molecule is a measure of the extent to which an applied electric
field induces an electric dipole (Topic H6). The anisotropy is the variation of the
polarizability with the orientation of the molecule relative to the electromagnetic field of
the radiation. Entirely spherically symmetric molecules such as tetrahedral CH 4 or
octahedral SF 6 have the same polarizability regardless of orientation so these molecules
are rotationally Raman inactive. All non-spherically symmetric molecules are
rotationally Raman active.
The specific selection rules for allowed rotational Raman transitions of linear
molecules are:
∆J=+2 (Stokes lines) ∆J=−2 (anti-Stokes lines)
so the energies corresponding to allowed transitions are given by:
∆E−E J+2−E J=B(J+2)(J+3)−BJ(J+1)=2B(2J+3)
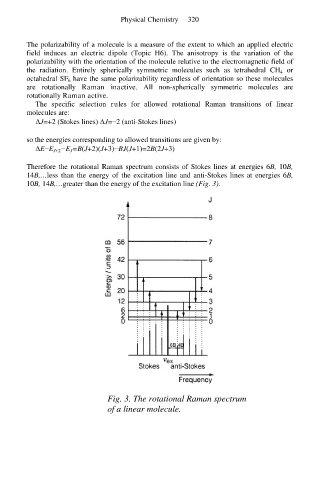
Therefore the rotational Raman spectrum consists of Stokes lines at energies 6B, 10B,
14B,…less than the energy of the excitation line and anti-Stokes lines at energies 6B,
10B, 14B,…greater than the energy of the excitation line (Fig. 3).
Fig. 3. The rotational Raman spectrum
of a linear molecule.