Page 333 - Instant notes
P. 333
Rotational spectroscopy 319
giving rise to the transition of maximum intensity) is obtained by differentiating the
above expression and setting this equal to zero:
and is dependent on the temperature and the magnitude of B. (Note that the units of B
must be the same as the units of k BT when substituting numerical values.)
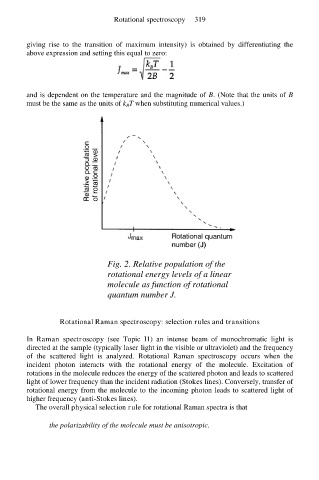
Fig. 2. Relative population of the
rotational energy levels of a linear
molecule as function of rotational
quantum number J.
Rotational Raman spectroscopy: selection rules and transitions
In Raman spectroscopy (see Topic I1) an intense beam of monochromatic light is
directed at the sample (typically laser light in the visible or ultraviolet) and the frequency
of the scattered light is analyzed. Rotational Raman spectroscopy occurs when the
incident photon interacts with the rotational energy of the molecule. Excitation of
rotations in the molecule reduces the energy of the scattered photon and leads to scattered
light of lower frequency than the incident radiation (Stokes lines). Conversely, transfer of
rotational energy from the molecule to the incoming photon leads to scattered light of
higher frequency (anti-Stokes lines).
The overall physical selection rule for rotational Raman spectra is that
the polarizability of the molecule must be anisotropic.