Page 356 - Instant notes
P. 356
Physical Chemistry 342
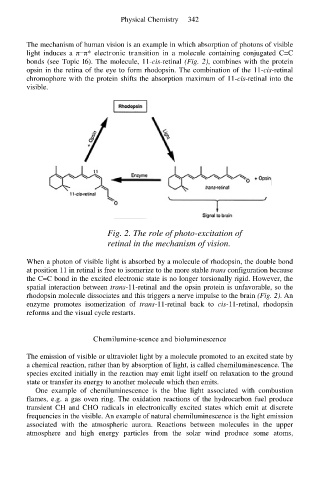
The mechanism of human vision is an example in which absorption of photons of visible
light induces a π−π* electronic transition in a molecule containing conjugated C=C
bonds (see Topic I6). The molecule, 11-cis-retinal (Fig. 2), combines with the protein
opsin in the retina of the eye to form rhodopsin. The combination of the 11-cis-retinal
chromophore with the protein shifts the absorption maximum of 11-cis-retinal into the
visible.
Fig. 2. The role of photo-excitation of
retinal in the mechanism of vision.
When a photon of visible light is absorbed by a molecule of rhodopsin, the double bond
at position 11 in retinal is free to isomerize to the more stable trans configuration because
the C=C bond in the excited electronic state is no longer torsionally rigid. However, the
spatial interaction between trans-11-retinal and the opsin protein is unfavorable, so the
rhodopsin molecule dissociates and this triggers a nerve impulse to the brain (Fig. 2). An
enzyme promotes isomerization of trans-11-retinal back to cis-11-retinal, rhodopsin
reforms and the visual cycle restarts.
Chemilumine-scence and bioluminescence
The emission of visible or ultraviolet light by a molecule promoted to an excited state by
a chemical reaction, rather than by absorption of light, is called chemiluminescence. The
species excited initially in the reaction may emit light itself on relaxation to the ground
state or transfer its energy to another molecule which then emits.
One example of chemiluminescence is the blue light associated with combustion
flames, e.g. a gas oven ring. The oxidation reactions of the hydrocarbon fuel produce
transient CH and CHO radicals in electronically excited states which emit at discrete
frequencies in the visible. An example of natural chemiluminescence is the light emission
associated with the atmospheric aurora. Reactions between molecules in the upper
atmosphere and high energy particles from the solar wind produce some atoms,