Page 435 - Instrumentation Reference Book 3E
P. 435
418 Chemical analysis: moisture measurement
of such sensors is that as no imposed gas flow contamination of the two crystals should be simi-
is necessary; they can simply be placed in the lar, and the frequency difference little affected,
gas to be measured, for example, an environ- resulting in stability. However, regular calibra-
mental chamber. In addition, they can be used tion is still necessary, and the complexity of the
at high pressure, they have a wide response instrument makes it expensive.
range (typically 50°C to -80°C dew point for
a single aluminum oxide sensor), have a rapid
response, and are generally not expensive. 19.3.1.6 Automatic psychrometers
These advantages often outweigh any problems The measurement of the temperature difference
of drift and stability, and the requirement for between a dry thermometer bulb and one sur-
regular calibration, but they must be used rounded by a wet muslin bag fed by a wick is
with care. the classical meteorological humidity measure-
ment. This is called psychometry, and automated
instrunients are available. The rate of evapor-
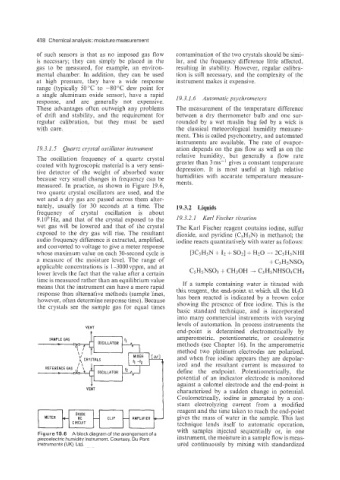
19.3.1.5 Quartz crystal oscillator instrument ation depends on the gas flow as well as on the
relative humidity, but generally a flow rate
The oscillation frequency of a quartz crystal greater than 3 ms-' gives a constant temperature
coated with hygroscopic material is a very sensi-
tive detector of the weight of absorbed water depression. It is most useful at high relative
humidities with accurate temperature measure-
because very small changes in frequency can be
measured. In practice, as shown in Figure 19.6, ments.
two quartz crystal oscillators are used, and the
wet and a dry gas are passed across them alter-
nately, usually for 30 seconds at a time. The 19.3.2 Liquids
frequency of crystal oscillation is about
9.106Hz, and that of the crystal exposed to the 19.3.2. I Karl Fischer titration
wet gas will be lowered and that of the crystal The Karl Fischer reagent contains iodine, sulfur
exposed to the dry gas will rise. The resultant dioxide, and pyridine (C5H5N) in methanol; the
audio frequency difference is extracted, amplified, iodine reacts quantitatively with water as follows:
and converted to voltage to give a meter response
whose maximum value on each 30-second cycle is [~C~HSN
+ I2 + SO21 + H20 + 2C5HjNHI
a measure of the moisture level. The range of + C5HjNS03
applicable concentrations is 1-3000 vppm, and at
lower levels the fact that the value after a certain C5H5NSOj + CH30H + C~HSNHSO~CH~
time is measured rather than an equilibrium value
means that the instrument can have a more rapid If a sample containing water is titrated with
response than alternative methods (sample lines, this reagent, the end-point at which all the H20
however, often determine response time). Because has been reacted is indicated by a brown color
showing the presence of free iodine. This is the
the crystals see the sample gas for equal times
basic standard technique, and is incorporated
into many commercial instruments with varying
levels of automation. In process instruments the
VENT end-point is determined electrometrically by
- OSCILLATOR methods (see Chapter 16). In the amperometric
SAMPLE GAS '' amperometric. potentiometric, or coulometric
method two platinum electrodes are polarized,
): CRYSTALS and when free iodine appears they are depolar-
REFERENCE GAS 1 \ f2 fiv 1 ized and the resultant current is measured to
OSCILLATOR define the endpoint. Potentiometrically, the
potential of an indicator electrode is monitored
against a calomel electrode and the end-point is
VENT characterized by a sudden change in potential.
Coulometrically, iodine is generated by a con-
stant electrolyzing current from a modified
reagent and the time taken to reach the end-point
DIODE
METER + RC - CLIP - AMPLIFIER gives the mass of water in the sample. This last
CIRCUIT technique lends itself to automatic operation,
with samples injected sequentially or, in one
Figure 19.6 A blockdiagram of thearrangement of a
piezoelectric humidity instrument. Courtesy, Du Pont instrument, the moisture in a sample flow is meas-
Instruments (UK) Ltd. ured continuously by mixing with standardized