Page 432 - Instrumentation Reference Book 3E
P. 432
Definitions 415
given by the volume concentration multiplied by
the molecular weight of water and divided by that
of the carrier gas. Meteorologists often call the
weight concentration the "mixing ratio" and
express it in gtkg.
When the prime aim is to avoid condensation
the appropriate unit is the dew point, which is the
temperature at which the vapor pressure of the
moisture would become szturated with respect to
a plane surface. Similarly the frost point refers io
the formation of ice. The relationship between
dew and frost points and saturated vapor pres-
sure is derived from thermodynamic and expesi-
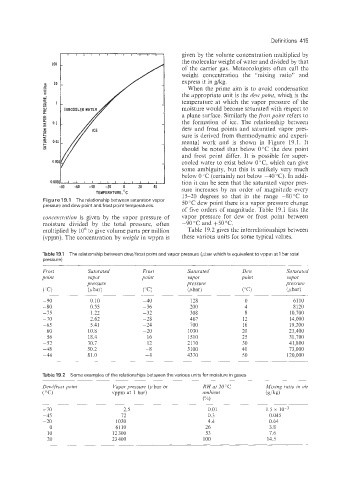
mental work and is shown in Figure 19.1. It
should be noted that below 0°C the dew point
and frost point differ. It is possible for super-
cooled water to exist below O"G, which can give
some ambiguity, but this is unlikely very much
below 0 "C (certainly not below -40°C). In addi-
I 1
0.0001L-1 I I I I I I I I I tion it can be seen that the saturated vapor pres-
I
-80 -60 -40 -20 0 20 40 sure increases by an order of magnitude every
TEMPERATURE.'C
15-20 degrees so that in the range -80°C to
Figure 19.1 The relationship between saturation vapor 50 "C dew point there is a vapor pressure change
pressure and dew point and frost point temperatures.
of five orders of magnitude. Table 19.1 lists the
coiicentration is given by the vapor pressure of vapor pressure for dew or frost point between
moisture divided by the total pressure, often -90 "C and +50 "C.
multiplied by IO6 to give volume parts per million Table 19.2 gives the interrelationships between
(vppm). The concentration by weight in wppm is these various units for some typical values.
Table 19.1 The relationship between dew/frost point and vapor pressure (pbar which is equivalent bo vppm at 1 bar total
pressure)
Frost Suturated Frosr Saturated Dew Saturated
point vapor point vapor point vapor
pressure pressure pressure
( 'C) (bar) ("C) WJar) ("C) War)
-90 0.10 -40 128 0 61 10
-80 0.55 -36 200 4 8120
-75 1.22 -32 308 8 10,700
-70 2.62 -28 467 12 14,000
-65 5.41 -24 700 16 19,200
-60 10.8 -20 1030 20 23,400
-56 18.4 -16 1510 25 31,700
-52 30.7 -12 2170 30 41,800
-48 50.2 -8 3100 40 73,000
-44 81.0 -4 4370 50 120,000
Taabie 19.2 Some examples of the relationships between the various units for moisture in gases
Dewyrost point Vapor pressure (p bar or RH at 20 "C Mixing ratio in air
("C) vppm at 1 bar) ambient (gIk-3)
("10)
-70 2.5 0.01 1.5 x
-45 72 0.3 0.045
-20 1030 4.4 0.64
0 61 10 26 3.8
10 12 300 53 7.6
20 23 400 100 14.5