Page 427 - Instrumentation Reference Book 3E
P. 427
410 Chemical analysis: gas analysis
Ambient air E 0 Converter
3
I Zero calibration
/ Dual head solenoid Reactor
-V-l
( pump 1
Span gas- '\ I
\
Sample in
Calibration Pressure
solenoid gauge r
I 1
\ Flowmeter
Back pressure
regulator
Exhaust gas
I 3 I
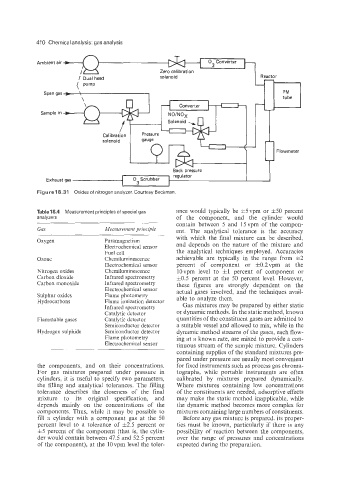
Figure 18.31 Oxides of nitrogen analyzer. Courtesy Beckman
Table 18.4 Measurement principles of special gas ance would typically be +5vpm or f50 percent
analyzers of the component, and the cylinder would
contain between 5 and 15 vpm of the compon-
Gus Measurement principle ent. The analytical tolerance is the accuracy
~ with which the final mixture can be described,
Oxygen Paramagnetism
Electrochemical sensor and depends on the nature of the mixture and
Fuel cell the analytical techniques employed. Accuracies
Ozone Chemiluminescence achievable are typically in the range from 52
Electrochemical sensor percent of component or f0.2vpm at the
Nitrogen oxides Chemiluminescence lOvpm level to +1 percent of component or
Carbon dioxide Infrared spectrometry +OS percent at the 50 percent level. However,
Carbon monoxide Infrared spectrometry these figures are strongly dependent on the
Electrochemical sensor actual gases involved, and the techniques avail-
Sulphur oxides Flame photometry able to analyze them.
Hydrocarbons Flame ionization detector
Infrared spectrometry Gas mixtures may be prepared by either static
Catalytic detector or dynamic methods. In the static method, known
Flammable gases Catalytic detector quantities of the constituent gases are admitted to
Semiconductor detector a suitable vessel and allowed to mix, while in the
Hydrogen sulphide Semiconductor detector dynamic method streams of the gases, each flow-
Flame photometry ing at a known rate, are mixed to provide a con-
Electrochemical sensor tinuous stream of the sample mixture. Cylinders
containing supplies of the standard mixtures pre-
pared under pressure are usually most convenient
the components, and on their concentrations. for fixed instruments such as process gas chroma-
For gas mixtures prepared under pressure in tographs, while portable instruments are often
cylinders, it is useful to specify two parameters, calibrated by mixtures prepared dynamically.
the filling and analytical tolerances. The filling Where mixtures containing low concentrations
tolerance describes the closeness of the final of the constituents are needed, adsorptive effects
mixture to its original specification, and may make the static method inapplicable, while
depends mainly on the concentrations of the the dynamic method becomes more complex for
components. Thus, while it may be possible to mixtures containing large numbers of constituents.
fill a cylinder with a component gas at the 50 Before any gas mixture is prepared, its proper-
percent level to a tolerance of 4~2.5 percent or ties must be known, particularly if there is any
&5 percent of the component (that is, the cylin- possibility of reaction between the components,
der would contain between 47.5 and 52.5 percent over the range of pressures and concentrations
of the component), at the 10 vpm level the toler- expected during the preparation.