Page 423 - Instrumentation Reference Book 3E
P. 423
406 Chemical analysis: gas analysis
But for a gas, the density is proportional to llT per Kelvin. This can be automatically compen-
where Tis the absolute temperature. Thus sated by a resistance thermometer placed in the
gas stream near the cell. The calibration depends
C
volume susceptibility = on the pressure of the gas in the cell.
(T2 - 0T) Another error arises from the fact that the
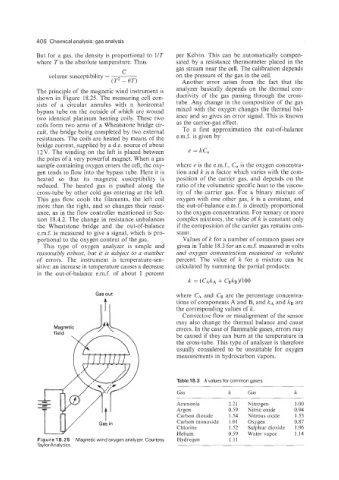
The principle of the magnetic wind instrument is analyzer basically depends on the thermal con-
shown in Figure 18.25. The measuring cell con- ductivity of the gas passing through the cross-
sists of a circular annulus with a horizontal tube. Any change in the composition of the gas
bypass tube on the outside of which are wound mixed with the oxygen changes the thermal bal-
two identical platinum heating coils. These two ance and so gives an error signal. This is known
coils form two arms of a Wheatstone bridge cir- as the carrier-gas effect.
cuit, the bridge being completed by two external To a first approximation the out-of-balance
resistances. The coils are heated by means of the e.m.f. is given by
bridge current, supplied by a d.c. source of about
12V. The winding on the left is placed between e = kC,
the poles of a very powerful magnet. When a gas
sample containing oxygen enters the cell, the oxy- where e is the e.m.f., C, is the oxygen concentra-
gen tends to flow into the bypass tube. Here it is tion and k is a factor which varies with the com-
heated so that its magnetic susceptibility is position of the carrier gas, and depends on the
reduced. The heated gas is pushed along the ratio of the volumetric specific heat to the viscos-
cross-tube by other cold gas entering at the left. ity of the carrier gas. For a binary mixture of
This gas flow cools the filaments, the left coil oxygen with one other gas, k is a constant, and
more than the right, and so changes their resist- the out-of-balance e.m.f. is directly proportional
ance, as in the flow controller mentioned in Sec- to the oxygen concentration. For ternary or more
tion 18.4.2. The change in resistance unbalances complex mixtures, the value of k is constant only
the Wheatstone bridge and the out-of-balance if the composition of the carrier gas remains con-
e.m.f. is measured to give a signal, which is pro- stant.
portional to the oxygen content of the gas. Values of k for a number of common gases are
This type of oxygen analyzer is simple and given in Table 18.3 for an e.m.f. measured in volts
reasonably robust, but it is subject to a number and oxygen concentration measured in volume
of errors. The instrument is temperature-sen- percent. The value of k for a mixture can be
sitive: an increase in temperature causes a decrease calculated by summing the partial products:
in the out-of-balance e.m.f. of about 1 percent
k = (CAkA + CBkB)/100
Gas out where CA and CB are the percentage concentra-
4 tions of components A and B, and kA and kg are
the corresponding values of k.
Convective flow or misalignment of the sensor
may also change the thermal balance and cause
errors. In the case of flammable gases, errors may
be caused if they can burn at the temperature in
the cross-tube. This type of analyzer is therefore
usually considered to be unsuitable for oxygen
measurements in hydrocarbon vapors.
Table 18.3 kvalues for common gases
Gas k Gas k
Ammonia 2.21 Nitrogen 1 .OO
Argon 0.59 Nitric oxide 0.94
Carbon dioxide 1.54 Nitrous oxide 1.53
Carbon monoxide 1.01 Oxygen 0.87
Chlorine 1.52 Sulphur dioxide 1.96
Helium 0.59 Water vapor 1.14
Figure 18.25 Magnetic wind oxygen analyzer. Courtesy Hydrogen 1.11
TaylorAnalytics.