Page 425 - Instrumentation Reference Book 3E
P. 425
408 Chemical analysis: gas analysis
controlled from the matched photocells upon
which the light from the mirror falls. In the Ser-
vomex instrument (Figure 18.29) the suspension
is platinum, and the restoring force is produced
electrically in a single turn of platinum wire con-
nected to the rest of the electronics through the
platinum suspension. Electromagnetic feedback
is used to maintain the dumb-bell in the zero
position, and the current required to do this is a
measure of the oxygen content of the gas.
The deflecting couple applied to the dumb-bell
by the magnetic field depends on the magnetic
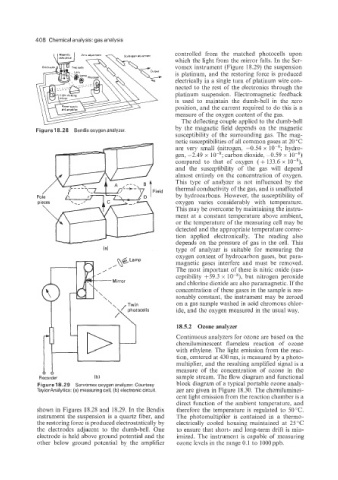
Figure 18.28 Bendix oxygen analyzer.
susceptibility of the surrounding gas. The mag-
netic susceptibilities of all common gases at 20 "C
are very small (nitrogen, -0.54 x lo@; hydro-
gen, -2.49 x lo-*; carbon dioxide, -0.59 x
compared to that of oxygen (f 133.6 x lo-'),
and the susceptibility of the gas will depend
almost entirely on the concentration of oxygen.
This type of analyzer is not influenced by the
thermal conductivity of the gas, and is unaffected
Field
by hydrocarbons. However, the susceptibility of
pieces oxygen varies considerably with temperature.
This may be overcome by maintaining the instru-
ment at a constant temperature above ambient,
or the temperature of the measuring cell may be
detected and the appropriate temperature correc-
tion applied electronically. The reading also
depends on the pressure of gas in the cell. This
type of analyzer is suitable for measuring the
oxygen content of hydrocarbon gases, but para-
magnetic gases interfere and must be removed.
The most important of these is nitric oxide (sus-
ceptibility +59.3 x lop8), but nitrogen peroxide
Mirror and chlorine dioxide are also paramagnetic. If the
concentration of these gases in the sample is rea-
sonably constant, the instrument may be zeroed
\ on a gas sample washed in acid chromous chlor-
ide, and the oxygen measured in the usual way.
18.5.2 Ozone analyzer
Continuous analyzers for ozone are based on the
chemiluminescent flameless reaction of ozone
with ethylene. The light emission from the reac-
tion, centered at 430 nni, is measured by a photo-
multiplier, and the resulting amplified signal is a
bb measure of the concentration of ozone in the
Recorder (b) sample stream. The flow diagram and functional
Figure 18.29 Servomexoxygenanalyzer. Courtesy block diagram of a typical portable ozone analy-
TaylorAnalytics: (a) measuring cell, (b) electronic circuit. zer are given in Figure 18.30. The chemilumines-
cent light emission from the reaction chamber is a
direct function of the ambient temperature, and
shown in Figures 18.28 and 18.29. In the Bendix therefore the temperature is regulated to 50°C.
instrument the suspension is a quartz fiber, and The photomultiplier is contained in a thermo-
the restoring force is produced electrostatically by electrically cooled housing maintained at 25 "C
the electrodes adjacent to the dumb-bell. One to ensure that short- and long-term drift is min-
electrode is held above ground potential and the imized. The instrument is capable of measuring
other below ground potential by the amplifier ozone levels in the range 0.1 to 1OOOppb.