Page 428 - Instrumentation Reference Book 3E
P. 428
Calibration of gas analyzers 411
18.6.1 Static methods induction period it is found that, provided the
tube is kept at constant temperature. the perme-
Static gas mixtures may be prepared either gravi- ation or diffusion rate is constant as long as there
metrically or by measurement of pressure. Since is liquid in the reservoir. The tube can then be
the weight of gas is usually small relative to the
weight of the cylinder required to contain it, gravi- calibrated gravimetrically to find the diffusion or
permeation rate of the sample. The concentration
metric procedures require balances, which have of the sample in the gas stream is then given by
both high capacity and high sensitivity, and the
buoyancy effect of the air displaced by the cylin- C = RKIF
der may be significant. Measurement of pressure
is often a more readily applicable technique. where C is the exit gas Concentration, R is the
After -preparation gas mixtures must be ad- diffusion or permeation rate, K is the reciprocal
equately mixed to ensure homogeneity, usually density of the sample vapor, and F is the gas flow
by prolonged continuous rolling of the cylinder. rate over the calibration cievice. The diffusion or
Once mixed, they should remain homogeneous permeation rate depends on the temperature of
over lon,g periods of time. Any concentration the tube, and on the molecular weight and vapor
changes are likely to be due to adsorption on pressure of the sample. Additionally, the diffu-
the cylinder walls. This is most likely to happen sion rate depends on the length and inner dia-
with mixtures containing vapors near their crit- meter of the capillary tube, and the permeation
ical pressures, and use of such mixtures should be rate depends on the nature, area, and thickness of
avoided if possible. the permeation membrane. Data are available for
Another common problem with complex sam- a large number of organic and inorganic vapors
ples is stratification over time, especially if they to allow tubes to be designed with the required
are stored in cooler ambient temperatures. One diffusion or permeation rate. and the exact rate
method to minimize this effect is to place heating for each tube is then established empirically.
blankets on the cylinders, which introduces ther- The temperature-dependence of diffusion or
mal currents in the bottles which keep the mixture permeation means that the tubes must be carefully
from “separating.” thermostatted for accurate calibrations. The empir-
ical equation for the temperature-dependence
of permeation rate is:
18.6.2 Dynamic methods
18.6.2 J GuslsJlow mixing
Gas mixtures of known concentration may be
prepared by mixing streams of two or more com-
ponents, each ofwhich is flowing at a known rate.
The concentration of one gas in the others may be
varied by adjustment of the relative flow rates,
but the range of concentration available is limited
by the range of flows, which can be measured
with sufficient accuracy. Electronic mass-flow
controllers are a convenient method of flow meas-
urement and control.
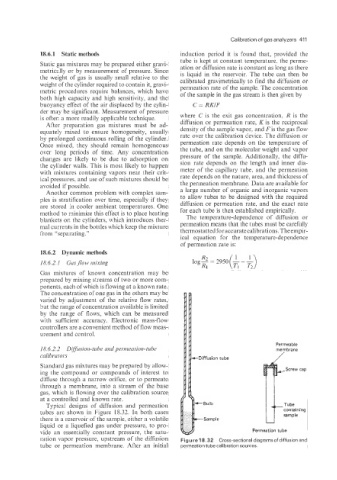
Permeable
18.6.2.2 Diffusion-tube and permeation-tube membrane
calibrcttoi,s -Diffusion tube
Standard gas mixtures may be prepared by allow-
ing the compound or compounds of interest to
diffuse through a narrow orifice, or to permeate
through a membrane, into a stream of the base
gas, which is flowing over the calibration source
at a controlled and known rate.
Typical designs of diffusion and permeation
tubes are shown in Figure 18.32. In both cases
there is a reservoir of the sample, either a volatile
liquid or a liquefied gas under pressure, to pro-
vide an essentially constant pressure, the satu- Permeation tube
ration vapor pressure, upstream of the diffusion Figure 18.32 Cross-sectional diagrams of diffusion and
tube or permeation membrane. After an initial permeation tube calibration sources.