Page 433 - Instrumentation Reference Book 3E
P. 433
41 6 Chemical analysis: moisture measurement
35 ments. To some extent the choice of technique
depends on the property required: dew point,
30
concentration or relative humidity. Only the
major techniques are discussed here. More exten-
sive treatments are given in the bibliography.
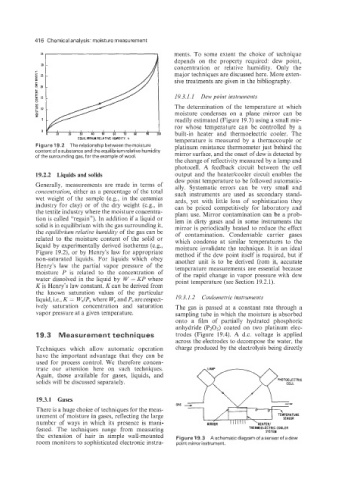
19.3.1.1 Dew point instruments
The determination of the temperature at which
moisture condenses on a plane mirror can be
readily estimated (Figure 19.3) using a small mir-
ror whose temperature can be controlled by a
a
built-in heater and thermoelectric cooler. The
temperature is measured by a thermocouple or
Figure 19.2 The relationship between the moisture platinum resistance thermometer just behind the
content of a substance and the equilibrium relative humidity mirror surface, and the onset of dew is detected by
of the surrounding gas, for the example of wool
the change of reflectivity measured by a lamp and
photocell. A feedback circuit between the cell
19.2.2 Liquids and solids output and the heaterlcooler circuit enables the
dew point temperature to be followed automatic-
Generally, measurements are made in terms of ally. Systematic errors can be very small and
concentration, either as a percentage of the total such instruments are used as secondary stand-
wet weight of the sample (e.g., in the ceramics ards, yet with little loss of sophistication they
industry for clay) or of the dry weight (e.g., in can be priced competitively for laboratory and
the textile industry where the moisture concentra- plant use. Mirror contamination can be a prob-
tion is called “regain”). In addition if a liquid or lem in dirty gases and in some instruments the
solid is in equilibrium with the gas surrounding it, mirror is periodically heated to reduce the effect
the equilibrium velutive humidity of the gas can be of contamination. Condensable carrier gases
related to the moisture content of the solid or which condense at similar temperatures to the
liquid by experimentally derived isotherms (e.g., moisture invalidate the technique. It is an ideal
Figure 19.2), or by Henry’s law for appropriate method if the dew point itself is required, but if
non-saturated liquids. For liquids which obey another unit is to be derived from it, accurate
Henry’s law the partial vapor pressure of the temperature measurements are essential because
moisture P is related to the concentration of of the rapid change in vapor pressure with dew
water dissolved in the liquid by W = KP where point temperature (see Section 19.2.1).
K is Henry’s law constant. K can be derived from
the known saturation values of the particular
liquid, i.e., K = WJP, where W, and P, are respect- 19.3.1.2 Coulometric instruments
ively saturation concentration and saturation The gas is passed at a constant rate through a
vapor pressure at a given temperature. sampling tube in which the moisture is absorbed
onto a film of partially hydrated phosphoric
anhydride (P205) coated on two platinum elec-
19.3 Measurement techniques trodes (Figure 19.4). A d.c. voltage is applied
across the electrodes to decompose the water, the
Techniques which allow automatic operation charge produced by the electrolysis being directly
have the important advantage that they can be
used for process control. We therefore concen-
trate our attention here on such techniques.
Again, those available for gases, liquids, and
solids will be discussed separately.
GAS -
19.3.1 Gases
There is a huge choice of techniques for the meas-
urement of moisture in gases, reflecting the large TEYPERATURE
SENSOR
number of ways in which its presence is mani- MIRROR HEATER/
fested. The techniques range from measuring THERMOELECTRIC COOLER
SYSTEM
the extension of hair in simple wall-mounted Figure 19.3 A schematic diagram of a sensor of a dew
room monitors to sophisticated electronic instru- point mirror instrument.