Page 111 - Introduction to Colloid and Surface Chemistry
P. 111
Liquid-gas and liquid-liquid interfaces 101
58 157
Surface r/ieo/ogy '
Surface viscosity is the change in the viscosity of the surface layer
brought about by the monomolecular film. Monolayers in different
physical states can readily be distinguished by surface viscosity
measurements,
A qualitative idea of the surface viscosity is given by noting the
ease with which talc can be blown about the surface. Most insoluble
l
6
1
3
films have surface viscosities of c. 10~ kg s~ to 10~ kg s" (for films
9 3
1(T m thick this is equivalent to a bulk viscosity range of c. 10 kg
m - 1 - 1 to 10 kg m s ). These films can be studied by means of a
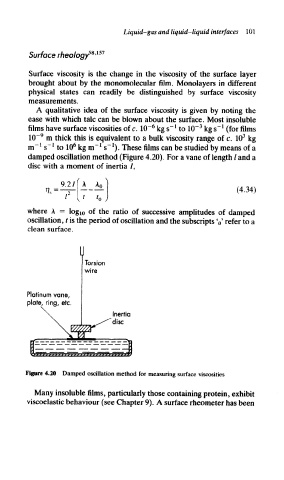
damped oscillation method (Figure 4.20). For a vane of length / and a
disc with a moment of inertia /,
(4.34)
where A = Iog 10 of the ratio of successive amplitudes of damped
oscillation, t is the period of oscillation and the subscripts V refer to a
clean surface.
U
Torsion
wire
Platinum vane,
plate, ring, etc.
Inertia
disc
Figure 4.20 Damped oscillation method for measuring surface viscosities
Many insoluble films, particularly those containing protein, exhibit
viscoelastic behaviour (see Chapter 9). A surface rheometer has been