Page 114 - Introduction to Colloid and Surface Chemistry
P. 114
104 Liquid-gas and liquid-liquid interfaces
1 TT
gives T = —= —
A kT
i.e. irA = kT (4.35)
where A is the average area per molecule.
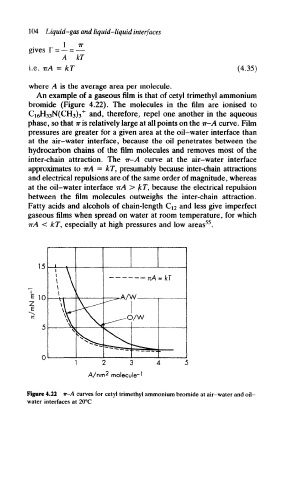
An example of a gaseous film is that of cetyl trimethyl ammonium
bromide (Figure 4.22). The molecules in the film are ionised to
C 16H 33N(CH3)3 + and, therefore, repel one another in the aqueous
phase, so that iris relatively large at all points on the it-A curve. Film
pressures are greater for a given area at the oil-water interface than
at the air-water interface, because the oil penetrates between the
hydrocarbon chains of the film molecules and removes most of the
inter-chain attraction. The it-A curve at the air-water interface
approximates to irA = kT, presumably because inter-chain attractions
and electrical repulsions are of the same order of magnitude, whereas
at the oil-water interface IT A > kT, because the electrical repulsion
between the film molecules outweighs the inter-chain attraction.
Fatty acids and alcohols of chain-length C^ and less give imperfect
gaseous films when spread on water at room temperature, for which
55
ttA < kT, especially at high pressures and low areas .
Figure 4.22 tt~A curves for cetyl trimethyl ammonium bromide at air-water and oil-
water interfaces at 20°C