Page 127 - Introduction to Colloid and Surface Chemistry
P. 127
The solid-gas interface 117
150
100
~9
o
</>
"O
a
a
CD
0.2 0.4 0.6 0.8 1.0
5
Pressure/10 N rrr 2
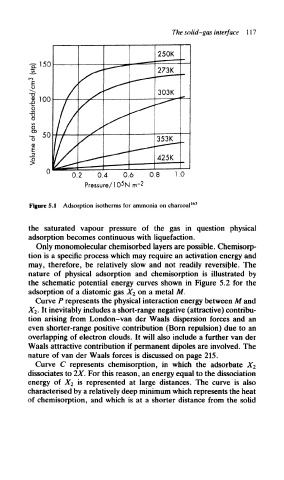
Figure 5.1 Adsorption isotherms for ammonia on charcoal 163
the saturated vapour pressure of the gas in question physical
adsorption becomes continuous with liquefaction.
Only monomolecular chemisorbed layers are possible. Chemisorp-
tion is a specific process which may require an activation energy and
may, therefore, be relatively slow and not readily reversible. The
nature of physical adsorption and chemisorption is illustrated by
the schematic potential energy curves shown in Figure 5.2 for the
adsorption of a diatomic gas X 2 on a metal M.
Curve P represents the physical interaction energy between M and
X 2, It inevitably includes a short-range negative (attractive) contribu-
tion arising from London-van der Waals dispersion forces and an
even shorter-range positive contribution (Born repulsion) due to an
overlapping of electron clouds. It will also include a further van der
Waals attractive contribution if permanent dipoles are involved. The
nature of van der Waals forces is discussed on page 215.
Curve C represents chemisorption, in which the adsorbate X 2
dissociates to IX. For this reason, an energy equal to the dissociation
energy of X 2 is represented at large distances. The curve is also
characterised by a relatively deep minimum which represents the heat
of chemisorption, and which is at a shorter distance from the solid