Page 128 - Introduction to Colloid and Surface Chemistry
P. 128
! 18 The solid-gas interface
X + X
Dissociation
energy of X2
Activation energy
for chemisorption
p Distance
Heat of
physical adsorption
Heat of
chemisorption
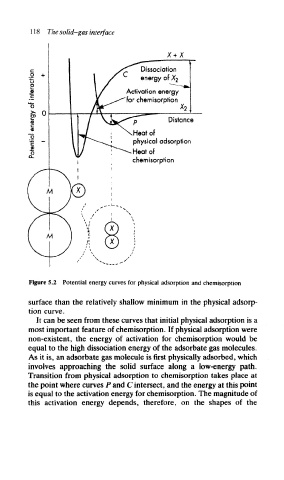
Figure 5.2 Potential energy curves for physical adsorption and chemisorption
surface than the relatively shallow minimum in the physical adsorp-
tion curve.
It can be seen from these curves that initial physical adsorption is a
most important feature of chemisorption. If physical adsorption were
non-existent, the energy of activation for chemisorption would be
equal to the high dissociation energy of the adsorbate gas molecules.
As it is, an adsorbate gas molecule is first physically adsorbed, which
involves approaching the solid surface along a low-energy path.
Transition from physical adsorption to chemisorption takes place at
the point where curves P and C intersect, and the energy at this point
is equal to the activation energy for chemisorption. The magnitude of
this activation energy depends, therefore, on the shapes of the