Page 133 - Introduction to Colloid and Surface Chemistry
P. 133
The solid—gas interface 123
diameters usually being between 2 nm and 20 nm. The upper limit of
adsorption is mainly governed by the total pore volume.
Types III (e.g. bromine on silica gel at 352 K) and V (e.g. water
vapour on charcoal at 373 K) show no rapid initial uptake of gas, and
occur when the forces of adsorption in the first monolayer are
relatively small. These isotherms are rare.
Many adsorption isotherms are borderline cases between two or
more of the above types. In addition, there are some isotherms which
do not fit into Brunauer's classification at all, the most notable being
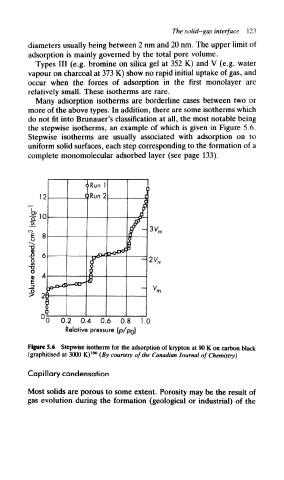
the stepwise isotherms, an example of which is given in Figure 5.6.
Stepwise isotherms are usually associated with adsorption on to
uniform solid surfaces, each step corresponding to the formation of a
complete monomolecular adsorbed layer (see page 133).
0.2 0.4 0.6 0.8 1.0
Relative pressure (p/po)
Figure 5.6 Stepwise isotherm for the adsorption of krypton at 90 K on carbon black
(graphitised at 3000 K) 166 (By courtesy of the Canadian Journal of Chemistry)
Capillary condensation
Most solids are porous to some extent. Porosity may be the result of
gas evolution during the formation (geological or industrial) of the