Page 137 - Introduction to Colloid and Surface Chemistry
P. 137
The solid-gas interface 127
p = 2y cos 91 r (5.4)
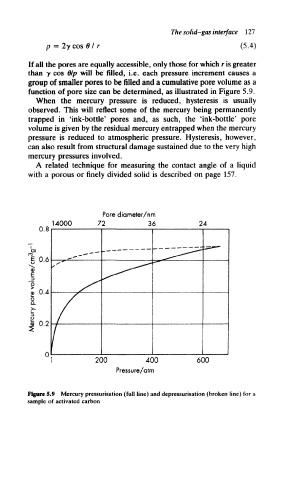
If all the pores are equally accessible, only those for which r is greater
than y cos 0/p will be filled, i.e. each pressure increment causes a
group of smaller pores to be filled and a cumulative pore volume as a
function of pore size can be determined, as illustrated in Figure 5.9.
When the mercury pressure is reduced, hysteresis is usually
observed. This will reflect some of the mercury being permanently
trapped in 'ink-bottle' pores and, as such, the 'ink-bottle' pore
volume is given by the residual mercury entrapped when the mercury
pressure is reduced to atmospheric pressure. Hysteresis, however,
can also result from structural damage sustained due to the very high
mercury pressures involved.
A related technique for measuring the contact angle of a liquid
with a porous or finely divided solid is described on page 157.
Pore diameter/nm
14000 72 36
200 400 600
Pressure/atm
Figure 5.9 Mercury pressurisation (full line) and depressurisation (broken line) for a
sample of activated carbon