Page 140 - Introduction to Colloid and Surface Chemistry
P. 140
130 The solid-gas interface
.2 1000
OL
800
600
O 400
TO
200
0.4 0.6 0.8
5 2
Pressure/ 10 Nm~
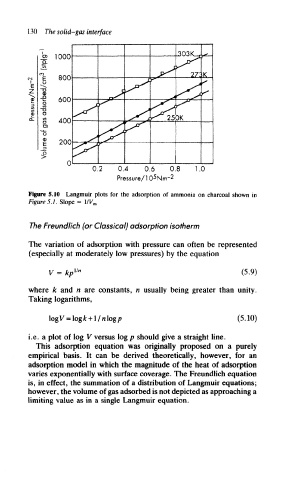
Figure 5.10 Langmuir plots for the adsorption of ammonia on charcoal shown in
Figures.!. Slope = l/V m
The Freundlich (or Classical) adsorption isotherm
The variation of adsorption with pressure can often be represented
(especially at moderately low pressures) by the equation
V = kp lln (5.9)
where k and n are constants, n usually being greater than unity.
Taking logarithms,
log V = log k +1 / n log p (5.10)
i.e. a plot of log V versus log p should give a straight line.
This adsorption equation was originally proposed on a purely
empirical basis. It can be derived theoretically, however, for an
adsorption model in which the magnitude of the heat of adsorption
varies exponentially with surface coverage. The Freundlich equation
is, in effect, the summation of a distribution of Langmuir equations;
however, the volume of gas adsorbed is not depicted as approaching a
limiting value as in a single Langmuir equation.