Page 143 - Introduction to Colloid and Surface Chemistry
P. 143
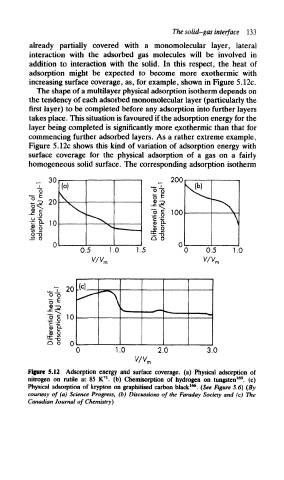
The solid-gas interface 133
already partially covered with a monomolecular layer, lateral
interaction with the adsorbed gas molecules will be involved in
addition to interaction with the solid. In this respect, the heat of
adsorption might be expected to become more exothermic with
increasing surface coverage, as, for example, shown in Figure 5.12c.
The shape of a multilayer physical adsorption isotherm depends on
the tendency of each adsorbed monomolecular layer (particularly the
first layer) to be completed before any adsorption into further layers
takes place. This situation is favoured if the adsorption energy for the
layer being completed is significantly more exothermic than that for
commencing further adsorbed layers. As a rather extreme example,
Figure 5.12c shows this kind of variation of adsorption energy with
surface coverage for the physical adsorption of a gas on a fairly
homogeneous solid surface. The corresponding adsorption isotherm
_ 30 200
J_ (a) T
o a E o
0
ll \ X 100
O
-«=
^x
<5
15-
I 9- "I- •*^^ "V, "•2 .2
10
M u* j= 8
OT3
J2 O o"o
0.5 1.0 1.5
_T 20 (c)
0~o \
o ^ t*^
\
^^ 10
"I 9.
<5 o
0^ 0
1.0 2.0 3.0
Figure 5.12 Adsorption energy and surface coverage, (a) Physical adsorption of
169
71
nitrogen on rutile at 85 K . (b) Chemisorption of hydrogen on tungsten , (c)
166
Physical adsorption of krypton on graphitised carbon black . (See Figure 5.6) (By
courtesy of (a) Science Progress, (b) Discussions of the Faraday Society and (c) The
Canadian Journal of Chemistry)