Page 142 - Introduction to Colloid and Surface Chemistry
P. 142
132 The solid-gas interface
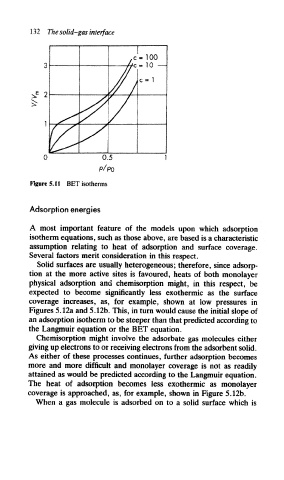
Figure 5.11 BET isotherms
Adsorption energies
A most important feature of the models upon which adsorption
isotherm equations, such as those above, are based is a characteristic
assumption relating to heat of adsorption and surface coverage.
Several factors merit consideration in this respect.
Solid surfaces are usually heterogeneous; therefore, since adsorp-
tion at the more active sites is favoured, heats of both monolayer
physical adsorption and chemisorption might, in this respect, be
expected to become significantly less exothermic as the surface
coverage increases, as, for example, shown at low pressures in
Figures 5.12a and 5.12b. This, in turn would cause the initial slope of
an adsorption isotherm to be steeper than that predicted according to
the Langmuir equation or the BET equation.
Chemisorption might involve the adsorbate gas molecules either
giving up electrons to or receiving electrons from the adsorbent solid.
As either of these processes continues, further adsorption becomes
more and more difficult and monolayer coverage is not as readily
attained as would be predicted according to the Langmuir equation.
The heat of adsorption becomes less exothermic as monolayer
coverage is approached, as, for example, shown in Figure 5.12b.
When a gas molecule is adsorbed on to a solid surface which is