Page 134 - Introduction to Colloid and Surface Chemistry
P. 134
124 The solid-gas interface
solid, it may reflect a fibrous structure, or it may be the result of
compaction of particulate solid.
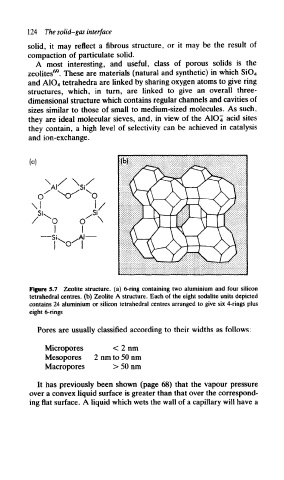
A most interesting, and useful, class of porous solids is the
69
zeolites . These are materials (natural and synthetic) in which SiO 4
and A1O 4 tetrahedra are linked by sharing oxygen atoms to give ring
structures, which, in turn, are linked to give an overall three-
dimensional structure which contains regular channels and cavities of
sizes similar to those of small to medium-sized molecules. As such,
they are ideal molecular sieves, and, in view of the A1C>4 acid sites
they contain, a high level of selectivity can be achieved in catalysis
and ion-exchange.
\
Si
X
0 \
1
—Sk
Figure 5.7 Zeolite structure, (a) 6-ring containing two aluminium and four silicon
tetrahedral centres, (b) Zeolite A structure. Each of the eight sodalite units depicted
contains 24 aluminium or silicon tetrahedral centres arranged to give six 4-rings plus
eight 6-rings
Pores are usually classified according to their widths as follows:
Micropores < 2 nm
Mesopores 2 nm to 50 nm
Macropores >50nm
It has previously been shown (page 68) that the vapour pressure
over a convex liquid surface is greater than that over the correspond-
ing flat surface. A liquid which wets the wall of a capillary will have a