Page 13 - Introduction to Colloid and Surface Chemistry
P. 13
4 The colloidal state
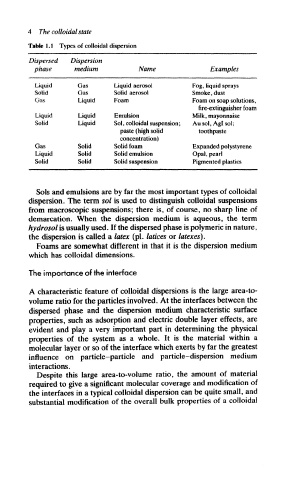
Table 1.1 Types of colloidal dispersion
Dispersed Dispersion
phase medium Name Examples
Liquid Gas Liquid aerosol Fog, liquid sprays
Solid Gas Solid aerosol Smoke, dust
Gas Liquid Foam Foam on soap solutions,
fire-extinguisher foam
Liquid Liquid Emulsion Milk, mayonnaise
Solid Liquid Sol, colloidal suspension; Ausol, Aglsol;
paste (high solid toothpaste
concentration)
Gas Solid Solid foam Expanded polystyrene
Liquid Solid Solid emulsion Opal, pearl
Solid Solid Solid suspension Pigmented plastics
Sols and emulsions are by far the most important types of colloidal
dispersion. The term sol is used to distinguish colloidal suspensions
from macroscopic suspensions; there is, of course, no sharp line of
demarcation. When the dispersion medium is aqueous, the term
hydrosol is usually used. If the dispersed phase is polymeric in nature,
the dispersion is called a latex (pi. latices or latexes}.
Foams are somewhat different in that it is the dispersion medium
which has colloidal dimensions.
The importance of the interface
A characteristic feature of colloidal dispersions is the large area-to-
volume ratio for the particles involved. At the interfaces between the
dispersed phase and the dispersion medium characteristic surface
properties, such as adsorption and electric double layer effects, are
evident and play a very important part in determining the physical
properties of the system as a whole. It is the material within a
molecular layer or so of the interface which exerts by far the greatest
influence on particle-particle and particle-dispersion medium
interactions.
Despite this large area-to-volume ratio, the amount of material
required to give a significant molecular coverage and modification of
the interfaces in a typical colloidal dispersion can be quite small, and
substantial modification of the overall bulk properties of a colloidal