Page 18 - Introduction to Colloid and Surface Chemistry
P. 18
The colloidal state 9
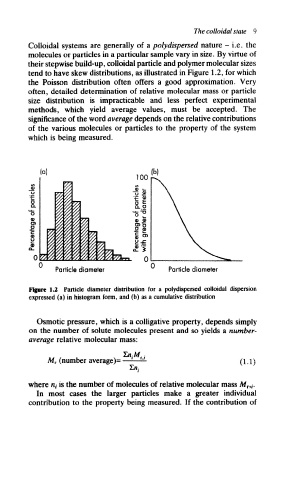
Colloidal systems are generally of a poly dispersed nature - i.e. the
molecules or particles in a particular sample vary in size. By virtue of
their stepwise build-up, colloidal particle and polymer molecular sizes
tend to have skew distributions, as illustrated in Figure 1.2, for which
the Poisson distribution often offers a good approximation. Very
often, detailed determination of relative molecular mass or particle
size distribution is impracticable and less perfect experimental
methods, which yield average values, must be accepted. The
significance of the word average depends on the relative contributions
of the various molecules or particles to the property of the system
which is being measured.
Particle diameter Particle diameter
Figure 1.2 Particle diameter distribution for a poiydispersed colloidal dispersion
expressed (a) in histogram form, and (b) as a cumulative distribution
Osmotic pressure, which is a colligative property, depends simply
on the number of solute molecules present and so yields a number-
average relative molecular mass:
M r (number average)= (LI)
where n/ is the number of molecules of relative molecular mass Af rn.
In most cases the larger particles make a greater individual
contribution to the property being measured. If the contribution of