Page 21 - Introduction to Colloid and Surface Chemistry
P. 21
12 The colloidal state
The rate of particle growth depends mainly on the following
factors:
1. The amount of material available.
2. The viscosity of the medium, which controls the rate of diffusion
of material to the particle surface.
3. The ease with which the material is correctly orientated and
incorporated into the crystal lattice of the particle.
4. Adsorption of impurities on the particle surface, which act as
growth inhibitors.
5. Particle-particle aggregation.
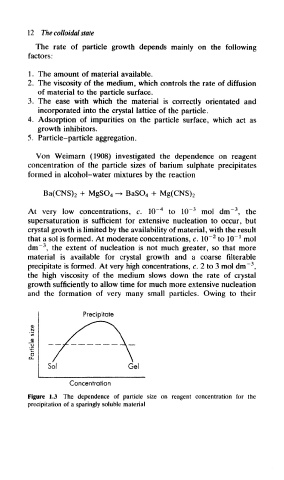
Von Weimarn (1908) investigated the dependence on reagent
concentration of the particle sizes of barium sulphate precipitates
formed in alcohol-water mixtures by the reaction
+ MgSO 4 -> +
Ba(CNS) 2 BaSO 4 Mg(CNS) 2
At very low concentrations, c. 10~ 4 to 10~ 3 mol dm~\ the
supersaturation is sufficient for extensive nucleation to occur, but
crystal growth is limited by the availability of material, with the result
1
2
that a sol is formed. At moderate concentrations, c. 10~ to 10" mol
3
dm"" , the extent of nucleation is not much greater, so that more
material is available for crystal growth and a coarse filterable
3
precipitate is formed. At very high concentrations, c. 2 to 3 mol dm" ,
the high viscosity of the medium slows down the rate of crystal
growth sufficiently to allow time for much more extensive nucleation
and the formation of very many small particles. Owing to their
Precipitate
Concentration
Figure 1.3 The dependence of particle size on reagent concentration for the
precipitation of a sparingly soluble material