Page 171 - Introduction to Colloid and Surface Chemistry
P. 171
160 The solid-liquid interface
agents also find considerable application in the textile industry for the
purpose of obtaining even results in operations such as scouring,
bleaching, mercerising and dyeing.
Cationic surfactants can be exploited to promote oil-wetting in
processes such as dry-cleaning and road making.
In addition to lowering y^, it is important that the wetting agent
s tnat
lowers L> ° a choice of surfactant to suit the particular nature
y S
of the solid surface has to be made (side-effects such as toxicity,
foaming, etc., must also be borne in mind). Irregularly shaped
surfactant molecules, e.g. sodium di-n-octyl sulphosuccinate (Aerosol
OT), are often very good wetting agents, since micelle formation is
not favoured, owing to steric considerations; this permits relatively
high concentrations of unassociated surfactant molecules and, hence,
greater lowering of y LG and y SL. Non-ionic surfactants are also good
wetting agents.
Water repel lency
This is the converse of the previous topic, the aim being to make the
contact angle as large as possible. Textile fabrics are made water-
repellent by treatment with a long-chain cationic surfactant (e.g. stear-
amidoniethylpyridinium chloride, C 17H 35CO NH CH 2N+C 5H 5 Cl~).
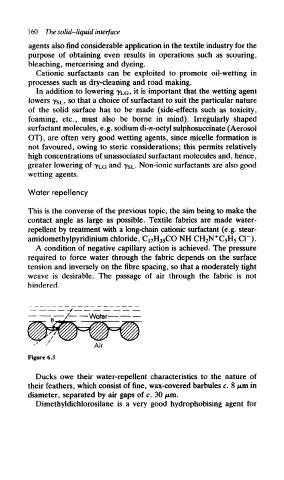
A condition of negative capillary action is achieved. The pressure
required to force water through the fabric depends on the surface
tension and inversely on the fibre spacing, so that a moderately tight
weave is desirable. The passage of air through the fabric is not
hindered.
Figure 6.5
Ducks owe their water-repellent characteristics to the nature of
their feathers, which consist of fine, wax-covered barbules c. 8 ^m in
diameter, separated by air gaps of c. 30 /u,m.
Dimethyldichlorosilane is a very good hydrophobising agent for