Page 172 - Introduction to Colloid and Surface Chemistry
P. 172
The solid-liquid interface 161
silica and glass surfaces; it reacts with the -OH groups on the outside
of the silicate lattice with the elimination of HCl to give
CHj CHj CHj CHj CH 3 CH 3 CH, CH,
\/ \/ \/ \/
Si Si Si Si
/ \ / \ / \ / \
0 0 0 o o o o o
l l i ' ! : l
O-Si-O-Si-O-Si-O-Si-O-Si-O-Si-O-Si-O-Si-O-
1 ! I I I \ \ I
Ore flotation 8081
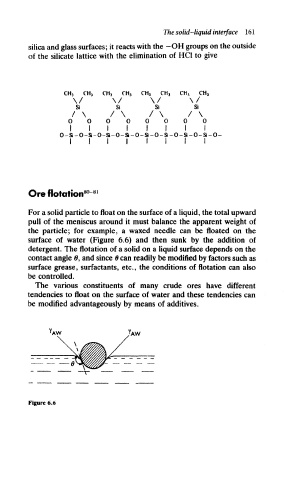
For a solid particle to float on the surface of a liquid, the total upward
pull of the meniscus around it must balance the apparent weight of
the particle; for example, a waxed needle can be floated on the
surface of water (Figure 6.6) and then sunk by the addition of
detergent. The flotation of a solid on a liquid surface depends on the
contact angle 6, and since 0 can readily be modified by factors such as
surface grease, surfactants, etc., the conditions of flotation can also
be controlled.
The various constituents of many crude ores have different
tendencies to float on the surface of water and these tendencies can
be modified advantageously by means of additives.
Figure 6.6